Despite the global pandemic, our team has been able to evolve from a laboratory-based diagnostic test developer to a full-service provider of clinical services based on the needs of the individuals and communities we serve. Our ability to adapt to changing conditions has simultaneously expanded our scope, increased patient access to high-quality care and delivered solutions which address large-scale public health issues.
A Team Effort
Quadrant has survived and thrived based on the collective energy, flexibility, creativity, and ingenuity of our entire team. As you learn more about Quadrant Biosciences, you may be compelled to characterize us as a telehealth company, a disruptive molecular diagnostics company, a behavioral health company, or even more broadly as an Autism company.
While we fit all those descriptions, none accurately captures the full scope of our operations and the power of our team.
Since our inception in 2015, we have chosen to do things
that are difficult and which have never been done before.
Our experience during the pandemic exemplified this: when else in history has a thinly capitalized company of 32 people grown so fast and accomplished so much? During the pandemic, we built two CLIA/CLEP clinical laboratories as well as a wastewater surveillance lab; we further developed a world-class R&D lab to create novel clinical assays using advanced, cutting-edge technologies. We built systems and software to better serve our clients. We started the process of building a Payor Relations team and appropriately expanded our Finance team. In short, we complemented our existing team with exceptional individuals throughout the company. All told, we developed arguably the world’s finest COVID test, achieved FDA authorization, and delivered our Clarifi COVID-19 test over 4 million times. Our wastewater surveillance team continues to build our business with geographic coverage of more than 90% of New York State and a growing capability to detect an array of human pathogens.
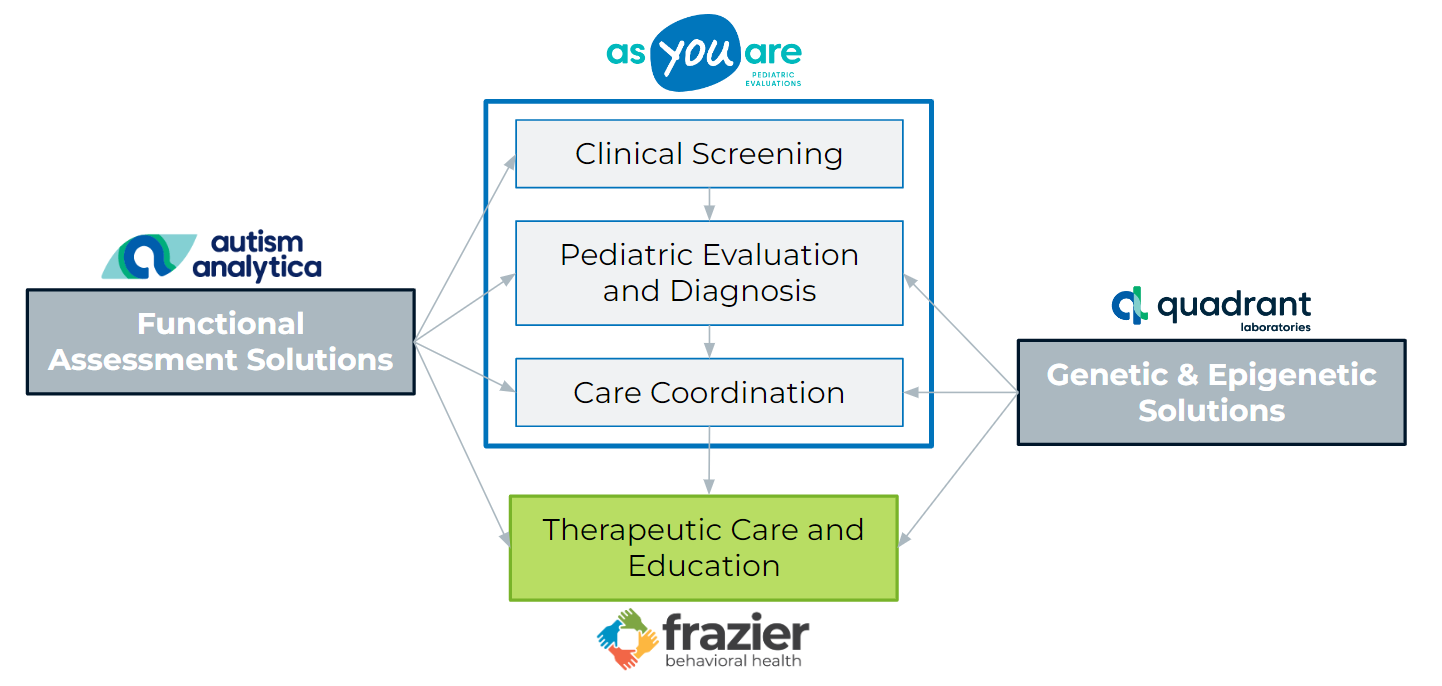
Addressing Problems in ASD Diagnosis
Starting over a year ago, we foresaw a gradual end to COVID testing and started to plan for the future of the company. We intentionally built on our pre-pandemic expertise in both mTBI and ASD, leveraging our battle-earned expertise.
With regard to ASD, we chose to solve two major problems facing children and families throughout the US.
1.) Delay in Diagnosis
In the US, the average delay from first clinical suspicion of ASD diagnosis is 3 years; compounding factors include:
- Limited access to expert diagnostic services
- Lack of objective clinical diagnostic tools
2.) Limited Clinical Capacity
Once diagnosed, clinical capacity for evidence-based therapeutic care is limited; compounding factors include:
- Minimal access to comprehensive “wrap around” services
- National shortage of clinical capacity
Finding Solutions to these Problems and Improving the Lives of Families
Despite the seemingly intractable nature of these problems, we believed at the time that Quadrant could resolve these issues and, in the process, improve the lives of hundreds of thousands of children and their families. In our assessment, we concluded that success would require multifactorial solutions, including expert services and transformative technologies


Management Team
Quadrant Biosciences houses a team of 150+ employees that is led by a highly experienced management team with expertise across functional areas. We are also supported by an advisory board comprised of highly regarded medical and scientific experts.