A simple, easy-to-use saliva test for COVID.
Quadrant’s COVID-19 PCR Saliva Test was developed by Quadrant Biosciences and SUNY Upstate Medical University.
Staying safe doesn’t have to be uncomfortable or complicated. Our saliva test is easy to use, painless, fast, and accurately detects Omicron, Delta, and all other known variants.
To date, this test has been used by over 2 million students, from kindergarten to college.
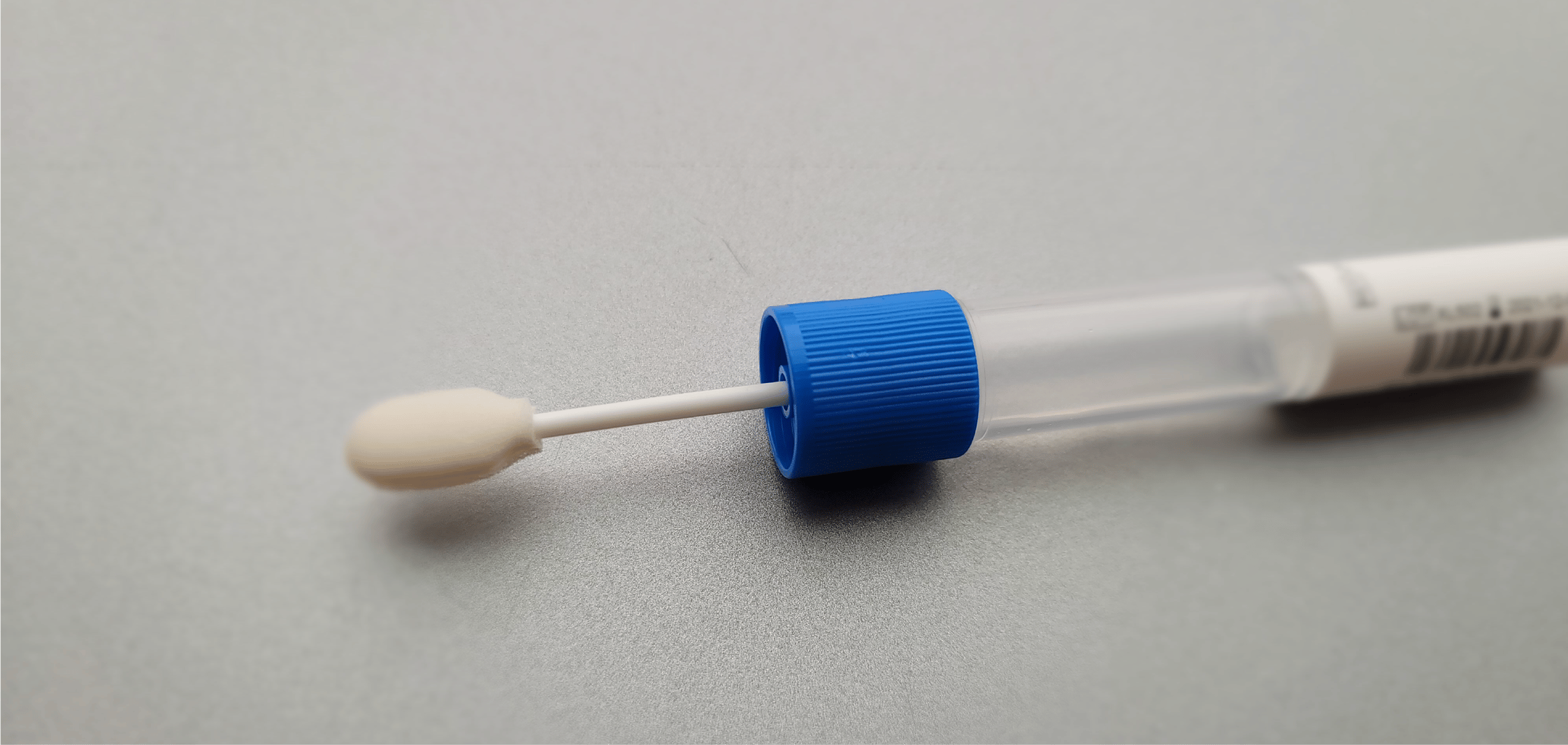
What is the swab made of?
The soft tip of the swab that goes in the mouth to collect saliva is made of polyurethane foam.
The handle is made of polypropylene homopolymer.
Ethylene Oxide is NOT contained in or used in the manufacturing process for the OR-100/ORE-100 saliva swab.
There are NO OTHER chemicals on the swab.

Is a PCR test accurate?
PCR tests are still the most sensitive tests for detecting the SARS-CoV-2 virus and will provide accurate and reliable results. But, not all PCR tests are able to detect the same concentrations of the virus.
The FDA requires that COVID test manufacturers measure the sensitivity of their test against their standardized SARS-CoV-2 Reference Panel².
The measure of this test sensitivity is known as Limit of Detection or LoD. LoD is reported as nucleic acid amplification test-detectable units (NDU) per milliliter or NDU/mL.
High LoDs will miss more infected patients and result in more false negatives. Low LoDs require less of the virus to be present, meaning they are more sensitive.
The LoD of our COVID-19 Saliva Test is 600, which is currently the lowest LoD for a saliva test with FDA EUA according to their reference panel.
Is this a diagnostic test?
Surveillance testing is diagnostic testing. Each test simply, reliably, and accurately will let you know if your child is positive for the SARS-CoV-2 virus. That knowledge empowers you to make good choices for the health and safety of your family.
Why do I have to register my child?
In order to use the COVID-19 PCR Saliva Test, you have to create an account on clarifi-covid-19.com and register your child. This is how all samples are tracked through our system and how results are reported to you.
You will have to register and verify your email and then create a profile, you can add your child or children under your profile. It is VERY important that you select your exact school name for your organization when you register.
Due to HIPAA compliance, you can’t delete an account once you create it.

Everything under one roof.
Our team does it all.
We co-developed the test with SUNY Upstate Medical University here in Syracuse.
Orders are placed with us, samples are sent to us, our labs (staffed by our employees) process your samples.
Results are reported by us, and we have live people in house to answer any questions you might have, every step of the way.
Our families live here, our children go to school here, our communities are important to us and we are honored to be a part of helping to keep them safe and to stop the spread of COVID-19.
If you still have questions you can click the button below to email us directly or you can follow this link to our FAQ’s.
*On September 22, 2020, the Clarifi® COVID-19 Test Kit obtained Emergency User Authorization (EUA) by the Food and Drug Administration (FDA) to be used for the diagnosis of SARS-COV-2. The Clarifi COVID-19 Test Letter of Authorization, along with the authorization Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients and authorized labeling are available on the FDA website.
The Clarifi COVID-19 Test Kit has not been FDA cleared or approved. It has been authorized by the FDA under an emergency use authorization for use by authorized laboratories. Clarifi COVID-19 has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens, and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
This test kit is not a direct to consumer test. This test kit is sold only to clinical laboratories in quantity.
Quadrant Laboratories, a subsidiary of Quadrant Biosciences, is a CLIA certified laboratory that provides individual and pooled COVID testing services ordered by a licensed healthcare provider using the Clarifi COVID Test Kit sold by Quadrant Biosciences.
Note: Testing is limited to qualified laboratories in the United States, certified under the Clinical Laboratory Improvement Amendment of 1988 (CLIA), 42 U.S.C. SS 263A, to perform high complexity testing.
References:
https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2#amendment
https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data#table2c